
9. One of the advantages of living on the top floor of a high rise is that you can get a good ____.
A. sight B. scene C. view D. look
8. I wrote him a letter to show my _____ of his thoughtfulness.
A. achievement B. agreement
C. attention D. appreciation
7. We look forward to the day when the motorcar has been replaced by some less dangerous ____ of transport.
A. methods B. means C. manners D. ways
6. ---- I’m sorry I stepped outside for a smoke. I was very tired.
---- There is no _____ for this while you are on duty.
A. reason B. excuse C. cause D. explanation
5. No regular advertiser dare produce anything that fails to stick to the ____ of his advertisement.
A. standard B. level C. text D. promise
4. I listened to Dr. Johnson’s lecture about biology, but I failed to get its key ____.
A. words B. notes C. messages D. points
3. ---- Hi, this way, please.
---- OK. I sometimes have no sense of ____ when I arrive at the crossroad.
A. position B. direction
C. situation D. condition
2. It has come to my _____ that some of you have been missing classes.
A. watch B. sight C. view D. notice
1. A month’s _____ is required from whichever party wishes to end the agreement.
A. message B. letter C. sentence D. notice
21.(09年山东理综·28)(14分)运用化学反应原理研究氮、氧等单质及其化合物的反应有重要意义。
(1)合成氨反应N2(g)+3H2(g) 2NH3(g),若在恒温、恒压条件下向平衡体系中通入氩气,平衡 移动(填“向左”、“向右”或“不”);使用催化剂 反应的△H(填“增大”、“减小”或“不改变”)。
2NH3(g),若在恒温、恒压条件下向平衡体系中通入氩气,平衡 移动(填“向左”、“向右”或“不”);使用催化剂 反应的△H(填“增大”、“减小”或“不改变”)。
(2)已知:O2(g) = O2+(g)+e-
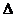 H1=1175.7
kJ·mol-1
H1=1175.7
kJ·mol-1
PtF6(g)+e-=PtF6-(g)
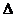 H2=-771.1
kJ·mol-1
H2=-771.1
kJ·mol-1
O2PtF6(S)=O2+(g)+PtF6-(g)
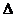 H3=482.2
kJ·mol-1
H3=482.2
kJ·mol-1
则反应O2(g)+PtF6(g) = O2+PtF6-(s)的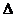 H=_____________
kJ·mol-1。
H=_____________
kJ·mol-1。
(3)在25℃下,向浓度均为0.1 mol·L-1的MgCl2和CuCl2混合溶液中逐滴加入氨水,先生成 沉淀(填化学式),生成该沉淀的离子方程式为 。已知25℃时Ksp[Mg(OH)2]=1.8×10-11,KsP[Cu(OH)2]=2.2×10-20。
(4)在25℃下,将a mol·L-1的氨水与0.01 mol·L-1的盐酸等体积混合,反应时溶液中c(NH4*)=c(Cl-)。则溶液显 性(填“酸”、“碱”或“中”);用含a的代数式表示NH3·H2O的电离常数Kb= 。
答案:(1)向左 不改变
(2)-77.6
(3)Cu(OH)2 Cu2++2NH3·H2O = Cu(OH)2↓ +2NH4+
(4)中 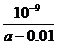 mol·L-1
mol·L-1
解析:(1)恒温、恒压条件下向平恒体系中通入氩气,则反应体系体积增大,平衡左移;使用催化剂只是改变了反应的途径,没有改变反应物与生成物的状态,△H不变;(2)利用盖斯定律,△H1+△H2+(-△H3)= -77.6 kJ·mol-1;(3)由于,KsP [Cu(OH)2]=2.2×10-20<Ksp[Mg(OH)2]=1.8×10-11,所以先生成沉淀;2NH3·H2O+Cu2+=Cu(OH)2↓+2 NH4*;根据溶液的电中性原则,c(NH4*)=c(Cl-),则[H+]=[OH-];溶液显中性;Kb= ,c(NH4*)=c(Cl-)=0.005 mol·L-1;[H+]=[OH-]=1×10-7 mol·L-1(因为是25℃下且为中性);[NH3·H2O]=mol·L-1-0.005 mol·L-1,则:Kb=mol·L-1。
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com