
9. _____ friend of my sister’s will come to see her tomorrow. I’m wondering what _____ man he is going to be like.
A. A; the B. A; a C. The; a D. The; the
8. You made the same mistake for _____ second time, dropping _____ “n” in the word “government”.
A. a; the B. a; a C. the; an D. a; an
7. ---- The news is spreading from mouth to mouth.
---- Yes, it’s become _____ talk of _____ town.
A. a; a B. the; / C. the; the D. a; /
6. ______will conquer ______ nature.
A. The man; / B. A man; the
C. Man; / D. The men; the
5. His brother likes ______ history very much. He knows a lot about ______Chinese history, especially ______history of social development.
A. /; / ; the B. / ; the ; /
C. the ; / ; / D. the ; / ; the
4. To tell you ______ truth, he is not _____ John I know before.
A. the; the B. a; a C. a ; the D. /; a
3. He is _____ honest man and now he is living in Belgium, _____ European country.
A. an; an B. a; an C. an; a D. an; the
2. Let us suppose that you are in____ position of____ parent. Would you allow your child to do such a thing?
A. a; a B. a; the C. the; a D. the; the
1. As is known to us all, _________ tiger is in _________ danger of becoming extinct.
A. the, a B. the, 不填 C. a,不填 D. 不填,the
19.(09年山东理综·10)下列关于氯的说法正确的是
A.Cl2具有很强的氧化性,在化学反应中只能作氧化剂
B.若35 17Cl、37 17若Cl为不同的核素,有不同的化学性质 学科
学科
C.实验室制备Cl2,可用排放和食盐水集气法收集
D.1.12LCl2含有1.7NA个质子(NA 表示阿伏伽德罗常数)
答案:C
解析:本题以氯元素为载体,从氧化还原、原子结构、收集方法不同角度进行考查,体现了山东理综化学选择题的命题模式。氯气与水的反应既是氧化剂也是还原剂;同一元素的不同核素化学性质几乎完全相同而物理性质不同;D中提Cl2的体积未指明是标准状况。实验室制备Cl2,可用排饱和食盐水集气法收集也可用瓶口向上排空气法收集,故C正确。
20 .(09年海南化学·8)下列叙述正确的是(用NA代表阿伏加德罗常数的值)
.(09年海南化学·8)下列叙述正确的是(用NA代表阿伏加德罗常数的值)
 A.2.4g金属镁变为镁离子时失去的电子数为0.1NA
A.2.4g金属镁变为镁离子时失去的电子数为0.1NA
 B.1molHCl气体中的粒子数与0.5 mo1/L盐酸中溶质粒子数相等
B.1molHCl气体中的粒子数与0.5 mo1/L盐酸中溶质粒子数相等
 C.在标准状况下,22.4LCH4与18gH2O所含有的电子数均为10 NA
C.在标准状况下,22.4LCH4与18gH2O所含有的电子数均为10 NA
 D.CO和N2为等电子体,22.4L的CO气体与lmol N2所含的电子数相等
D.CO和N2为等电子体,22.4L的CO气体与lmol N2所含的电子数相等
答案:C
解析:A中镁为0.1mol,失去的电子数为0.2 NA;B中盐酸无体积,不能计算出粒子数;D选项中使用气体摩尔体积不是在标准状况下。
21 .(09年海南化学·11)在5mL 0.05 mo1/L的某金属氯化物溶液中,滴加0.1 mo1/L AgNO3溶液,生成沉淀质量与加入AgNO3溶液体积关系如图所示,则该氯化物中金属元素的化合价为:
.(09年海南化学·11)在5mL 0.05 mo1/L的某金属氯化物溶液中,滴加0.1 mo1/L AgNO3溶液,生成沉淀质量与加入AgNO3溶液体积关系如图所示,则该氯化物中金属元素的化合价为:

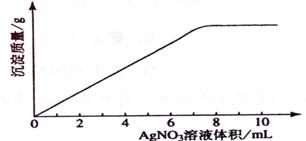
 A.+1 B.+2 C.+3 D.+4
A.+1 B.+2 C.+3 D.+4
答案:C
解析:设氯化物化学式为MClx
MClx -- x AgNO3
1 x
5mL×0.05 mol·L-1 0.1 mol·L-1×7.5mL
x = 3
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com