
27. Eating too much fat can______ heart disease and cause high blood pressure.
A. result from B. contribute to C. attend to D. lead in
26. You said boys were cleverer than girls. That is _______ I disagree.
A. what B. where C. which D. why
25. --- Nancy is not coming tonight. --- But she______!
A. promises B. promised C. will promise D. had promised
24. The bell______ the end of the period rang and we had to stop our discussion.
A. indicating B. indicated C. to be indicated D. being indicated
23. ---I hear John refused to tell the truth and was taken away by the police.
--- Where did you _______?
A. pick that up B. put that up C. make that up D. take that up
22. ---Are all telephone numbers_______ in the directory? --- Yes, all ________ Jane’s.
A. listed; including B. listed; included
C. including; includes D. being listed; being included
第一节、单项选择(15分)
21._____ we were worried about was _______ they could manage to control the pollution.
A. That; how B. That; whether C. What; that D. What; whether
4.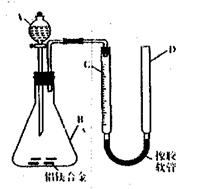 (湖北省武汉市教科院2009届高三第一次调考)某化学兴趣小组用下图所示装置测定铝镁合金薄片中铝质量分数和铝的相对原子质量。(铁架台等固定装置已略去)
(湖北省武汉市教科院2009届高三第一次调考)某化学兴趣小组用下图所示装置测定铝镁合金薄片中铝质量分数和铝的相对原子质量。(铁架台等固定装置已略去)
回答下列问题:
(1)实验前检查装置气密性的方法是 。
(2)实验前,先将铝镁合金薄片用砂纸打磨,其目的是 。
(3)A中试剂为 。
(4)经检查装置气密性完好后,将药品和水装入各仪器中,连接好装置,需进行的操作还有:①记录C中的液面位置;②将B中剩余固体过滤、洗涤、干燥、称重;③待B中还有气体产生,恢复至室温后,记录C的液面位置;④由A向B中滴加足量试剂。上述操作的顺序是 (填序号);记录C的液面位置时,除视线平视外,还应 。
(5)B中发生反应的化学方程式为 。
(6)若实验用铝镁合金薄片的质量为m g(打磨后称量),测得产生气体的体积为V ml.(已换算为标准状况),B中剩余固体的质量为n g,试计算铝的相对原子质量。
(7)合金薄片中铝的质量分数的表达式为 。
[解析](1)方法一:往装置D中加入一定量的水,关闭分液漏斗活塞,用手捂住装置B一段时间,如果装置C和装置D中形成稳定的液面差,说明装置不漏气,否则装置漏气。
方法二:先关闭漏斗活塞后,再向装置D中加入一定量的水,如果装置C和装置D中形成稳定的液面差,说明装置不漏气,否则装置漏气。
(2)除去铝镁合金表面的氧化膜。
(3)NaOH溶液(或KOH溶液或Ba(OH)2溶液)
(4)①④③② 使D和C的液面相平
(5)2Al+2NaOH+2H2O 2NaAlO2+3H2↑
(6)合金中合铝的质量为
铝与NaOH溶液反应析出氢气的物质的量为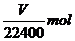
设合金中铝的物质的量为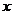
2Al+2NaOH+2H2O 2NaAlO2+3H2↑
2 3
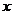
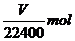
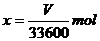
则铝的摩尔质量为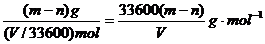
故铝的相对原子质量为
(7)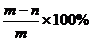 (答案为
(答案为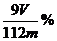 时,不给分)
时,不给分)
[答案](1)----(5)见解析,(6)  (7)
(7) 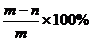
21世纪教育网
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com